Click and Bioorthogonal Chemistry
The 2022 Nobel Prize in Chemistry has been awarded jointly to professors K. Barry Sharpless, Morten Meldal, and Carolyn R. Bertozzi for their contributions in the fields of click chemistry and bioorthogonal chemistry, areas that have been revolutionary for drug synthesis and development, biomedical research, and nanotechnology.
The concept of “click chemistry” defines certain organic compound formation reactions, similar to those carried out by living organisms. A click reaction must be wide-ranging with very high degrees of conversion and selectivity, generating the minimum number of by-products. If they occur, they should be easily separable, without recourse to chromatographic separations, and should ideally be produced under sustainable conditions (preferably in water).
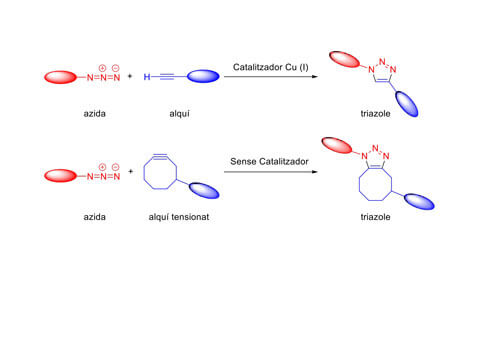
There are many examples of click reactions. The best known one is called click reaction, which is the cycloaddition reaction between an azide and a terminal alkyne catalysed by Cu (I), giving rise to the formation of cyclic triazoles. This reaction was reported simultaneously by Sharpless and Medal at the beginning of the century (2001-2002).
The term bioorthogonal, introduced by Dr Bertozzi in 2003, refers to a type of “click” chemistry that can be used in biological systems without altering them, only modifying the molecule of interest. Bioorthogonal reactions are the result of the development of metal-free “click” chemistries to avoid toxicity. These types of reactions have been used both to generate therapeutic proteins and to study the behaviour of certain biomolecules in complex biological systems such as cells and even animals.
IQS “click” applications
Different IQS research groups use these methodologies in their fields of expertise, such as biomedicine, nanotechnology, and the search for new drugs.
Within the Department of Organic and Pharmaceutical Chemistry at the IQS School of Engineering, specifically in the Pharmaceutical Chemistry Group (GQF), Dr Raimon Puig de la Bellacasa and Dr José I. Borrell are using “click” reactions with alkynes and azides in one of their lines of research to synthesize new candidates for drugs containing triazoles to treat adenocarcinoma of the pancreas, one of the types of cancer with the worst prognosis and worst survival rate for patients who suffer from it.
In the same department, Dr Ana Belén Cuenca’s research team is working on the development of marking probes to introduce 18F, based on ammonium trifluoborate type compounds, making it possible to study the in vivo biodistribution of drugs by PET technology (Positron Emission Tomography). This group uses both traditional “click” chemistry, catalysed by copper complexes (Cosialls et al., Chem. A. Eur. J. 2022), as well as the “bio-click” tool based on the use of stressed cycloalkynes.
In the Materials Engineering Group (GEMAT), Dr David Sanchez with the Supramolecular Chemistry Laboratory is leading a line of research in which they use “click” chemistry to prepare drug delivery systems based on hybrid nanoparticles (I. Pontón, D. Sanchez-García, J. Porphyr. Phthalocyanines 2021, 25, 917-929).
In the Bioengineering Department, the team led by Dr Benjamí Oller Salvia with the Therapeutic Protein Laboratory is using both methodologies to bind peptides and proteins, especially antibodies, to drugs and nanoparticles to aim them at target cells (Angew. Chem. Int. Ed. 2018, 57, 2831; Angew. Chem. Int. Ed. 2019, 58, 10844). With this chemistry, they can control how they bind proteins and maximize their efficiency with great precision. Currently, they are applying these reactions within the research projects they are conducting in the field of brain tumour treatment.


